Water behavior is influenced by temperature, pH, calcium hardness, alkalinity and TDS, with CYA also playing a key role in maintaining balance.

Keeping everything in balance is key to preventing problems like scaling or corrosion and ensuring the pool stays healthy for your enjoyment 24/7/365.
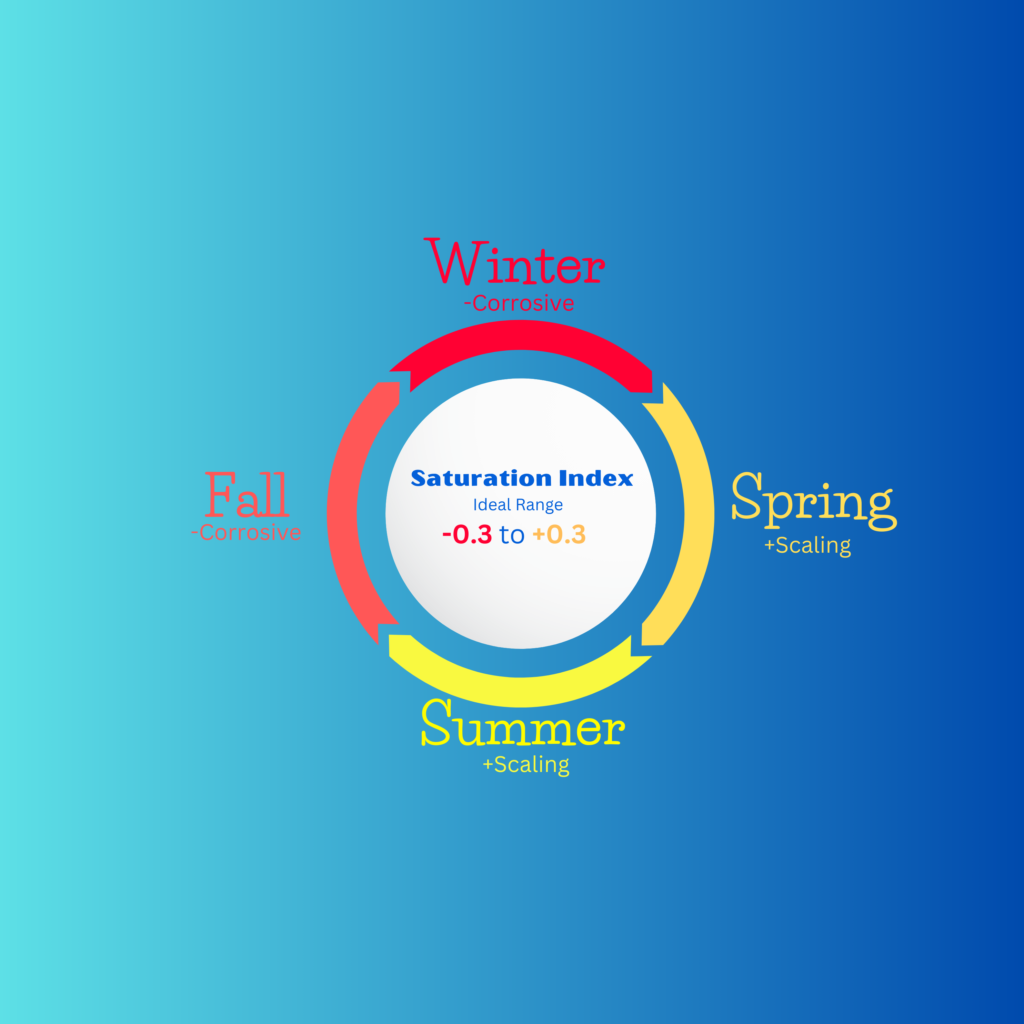
Corrosive vs. Scaling
In the Saturation Index negative means corrosive & positive means scaling
The most accurate way to read the Saturation Index is by using electronic devices specifically designed for water analysis. They provide precise readings and eliminate the room for human error such as water test strips or colorimetric methods.


Low temperatures tend to make the water corrosive and higher temperatures tend to make the water scaling. To reach optimal water balance year-round consider the following tips:

As temperatures begin to cool in the fall, it’s important to maintain total alkalinity and calcium hardness in the mid-to-high end of their ideal ranges to support balanced water chemistry. Keep pH toward the higher end of the range, around 7.8, by adding sodium bicarbonate, soda ash or naturally aerating the water with features like bubblers or fountains.

As water temperatures drop significantly, the risk of corrosive water conditions increases. To mitigate this, consider adding sodium bicarbonate to boost total alkalinity and calcium chloride to increase calcium hardness, both of which help shift water chemistry toward balance and reduce corrosion risk. Alternatively, natural aeration can be used to raise pH and alkalinity by releasing dissolved carbon dioxide from the water.

As water temperatures rise and pH fluctuations become more pronounced, adding muriatic acid or sodium bisulfate can help shift the water’s saturation balance towards negative LSI, reducing the risk of scaling. Draining and replacing water to lower calcium hardness is also recommended to prevent calcium carbonate buildup. Aim to maintain a pH between 7.2 and 7.5—not only to support balanced water chemistry but also to enhance chlorine’s efficiency at lower pH levels.

Warmer water temperatures often lead to elevated pH, which can trigger a chain reaction of scaling conditions, including high alkalinity and calcium hardness. To maintain proper balance, use pH reducers to shift water chemistry to the left and minimize calcium by-products. To help control pH and keep water balanced keep alkalinity low (under 80ppm), but if this approach is taken, keep calcium hardness in the high 400s. The key is keeping the pH ceiling low.
Range Chemistry
Range Chemistry serves as a base but it does not tell you the full story and using water test strips is merely a guess, not even close to an approximation. Digital water testing is a must to properly balance water
Langelier Saturation Index
LSI on the contrary; accounts for Actual pH, Temperature Factor, Calcium Hardness Factor, Total Alkalinity Factor and TDS Factor to give you a precise result
Consistent Water Balance